Abstract
Introduction
Patients with relapsed or refractory Diffuse large-B-cell lymphoma (RR DLBCL) have historically had limited treatment options. The approval of chimeric antigen receptor T-cell (CAR T) therapy in 2017 offered a significant survival benefit for patients with RR DLBCL beyond second line of therapy. While CAR T therapy is primarily administered in inpatient settings, anecdotal evidence suggests that outpatient CAR T therapy administration may be increasing as clinicians gain experience. We conducted an analysis of real-world CAR T treatment patterns to provide evidence on current CAR T use.
Methods
A retrospective analysis of the Anlitiks All-Payor Claims (AAPC) data for services rendered from April 2017 to December 2020 was conducted. The database includes fully adjudicated pharmacy and medical claims of patients that are insured through Medicare, Medicaid, or commercial plans covering over 80% of the US healthcare system. RR DLBCL patients (ICD-9/10-CMs 200.x, 202.8x; C83.3x, C84.6x, C84.7x, C85.2x) with a first claim (index date) for CAR T therapy (axicabtagene ciloleucel [axi-cel], tisagenlecleucel [tisa-cel], or unspecified CAR T agent) from October 2017 to September 2020, with ≥180 days of pre-index and ≥ 90 days of post-index follow-up were identified. Patient demographics, clinical characteristics, comorbidities, and treatment patterns including setting of CAR T infusion (inpatient/outpatient), time from leukapheresis to CAR T infusion, and incidence of CAR T-associated AEs and costs were analyzed. We utilized chi-square tests (categorical measures), t-tests, and Wilcoxon-Rank Sum tests (continuous measures) where appropriate. Logistic regression and Cox proportional hazards models were used to identify predictors of setting of CAR T infusion and time from leukapheresis to CAR T infusion.
Results
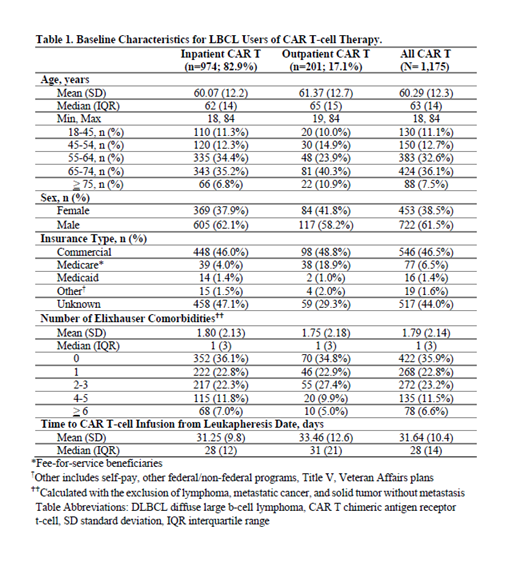
Of the 1,175 patients with RR DLBCL treated with CAR T therapy, 82.9% were infused inpatient. Overall, 61.5% (n=722) of patients were male, the mean age was 60.3 (±12.3) years, 46.5% (n=546) were commercially insured, 6.5% (n=77) had Medicare, 1.4% (n=16) had Medicaid, 44% (n=517) and 1.6% (n=19) had unknown and other insurance types, respectively. Outpatient CAR T infusion increased slightly from 15.9% in 2017 to 18.3% in 2018, then dropped to 17.2% in 2019 and 16.1% in 2020. Baseline patient characteristics are summarized in Table 1.
While median time from leukapheresis to CAR T infusion was similar for inpatient (28 days) and outpatient (31 days) settings, it was 24 days for axi-cel and 41 days for tisa-cel (p<0.001), nearly two times longer for the tisa-cel cohort as compared to the axi-cel cohort (HR: 2.04, 95% CI: 1.54-2.70). Medicare patients had a significantly lower likelihood of undergoing CAR T infusion in the inpatient setting (OR: 0.14, 95% CI: 0.08-0.23, p<0.001) compared to commercial, Medicaid, and patients with other insurance types.
Conclusions
In this claims-based analysis, more than 4 in 5 patients received CAR T therapy infusions in inpatient settings and the majority of patients with known insurance type were commercially insured. Axi-cel patients had a shorter time from leukapheresis to CAR T infusion compared to tisa-cel patients; opportunities to narrow this pre-treatment window still exist. Overall, there appears to be no clear trend in the pattern of CAR T infusion settings. Additional analyses on AE patterns and cost are underway to better understand therapeutic decision making.
Seyedin: Kite, A Gilead Company: Consultancy. Thornton Snider: Kite, a Gilead Company: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Gilead: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Rajagopalan: Kite, A Gilead Company: Consultancy. Wade: Amgen: Consultancy; Kite, A Gilead Company: Consultancy; Allergan: Consultancy. Gergis: Kite, A Gilead Company: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal